
RSC ESR 2023 – Giving EPR the Royal Treatment
April 4, 2023
Spin Class: EPR’s Long Distance Relationships
May 16, 2023
Insights from Austin
The latest insights on EPR technology trends, customer successes and industry best practises
Drug Discovery Chemistry 2023 – Context is King
Science is often about context. An outlier can only be determined in the context of the whole distribution. Experimental data is relevant only in the context of its setup and parameters. Biomolecules adopt different behaviors in the context of their local environment. Similarly, my previous Insights have examined EPR in the context of the broad biophysical (BPS) and the narrower EPR (RSC) communities. Most recently I attended Drug Discovery Chemistry 2023, and accordingly, this entry will be examining EPR in the context of the Drug Discovery workflow. Throughout the many excellent talks and presentations, a few key notes were played on refrain – notes that may sound good on an EPR instrument.
Starting from a broader context, a major area of interest is in-cell measurements on biological systems. This interest is not confined specifically to drug discovery, but is undeniably important in the process. As mentioned briefly above, biomolecules tend to exhibit high sensitivity to their local environments – a protein that adopts one conformation in the dilute environment of a sterile in-vitro buffer may adopt an entirely different conformation in the crowded, busy cellular milieu. While it is straightforward to understand that a potential drug in development must work in vivo just as it does in vitro, validating such activity may be anything but. EPR has in recent years enjoyed some excellent work on this precise problem, utilizing alternative spin labels based on gadolinium ions and trityl radicals to demonstrate distance measurements within cells. Such a technique may prove quicker and easier for a validation method than in-cell NMR or CryoEM, or may be used alongside them for deeper structural insights.
Another area of piqued interest is in ensemble measurements. Biomolecules are constantly in motion, from minor rotational/vibrational/translational modes to major conformational shifts. Capturing this motion is necessary for a complete understanding of the system – enabling more precision in designing drugs to target such biomolecules, and providing more insight into its activity (or lack thereof) on interaction with a potential drug. Crystallography forces a molecule into a lattice, providing a single, static structure, and NMR and CryoEM typically produce an averaged structure. EPR is by nature an ensemble technique, sampling all present conformations, and providing a probability distribution of distances. Such information coupled with an overall structural model can provide a deeper knowledge of the entire dynamic system of interest, and aid in the rational design of small molecule drugs.
Both of the above elements are relevant to drug discovery, but also are more general scientific concerns. To narrow in on more drug-discovery specific interests, we should discuss two hot topics in that field: conformational modulation of proteins, and ternary complexes in targeted protein degradation. Conformational modulation is closely associated with GPCRs – discussed briefly in my last post. In short, a GPCR or other protein is contacted by a ligand (small molecule, metal ion, etc.), whose binding to said protein alters the overall conformation. EPR is an ideal tool to assess such changes, giving fast and precise feedback and allowing easy iteration of ligand type, concentration, and other variables of interest. By monitoring the EPR distance distribution as a function of these variables one can map out the protein’s responses to various stimuli – a critical piece of information for drug development.
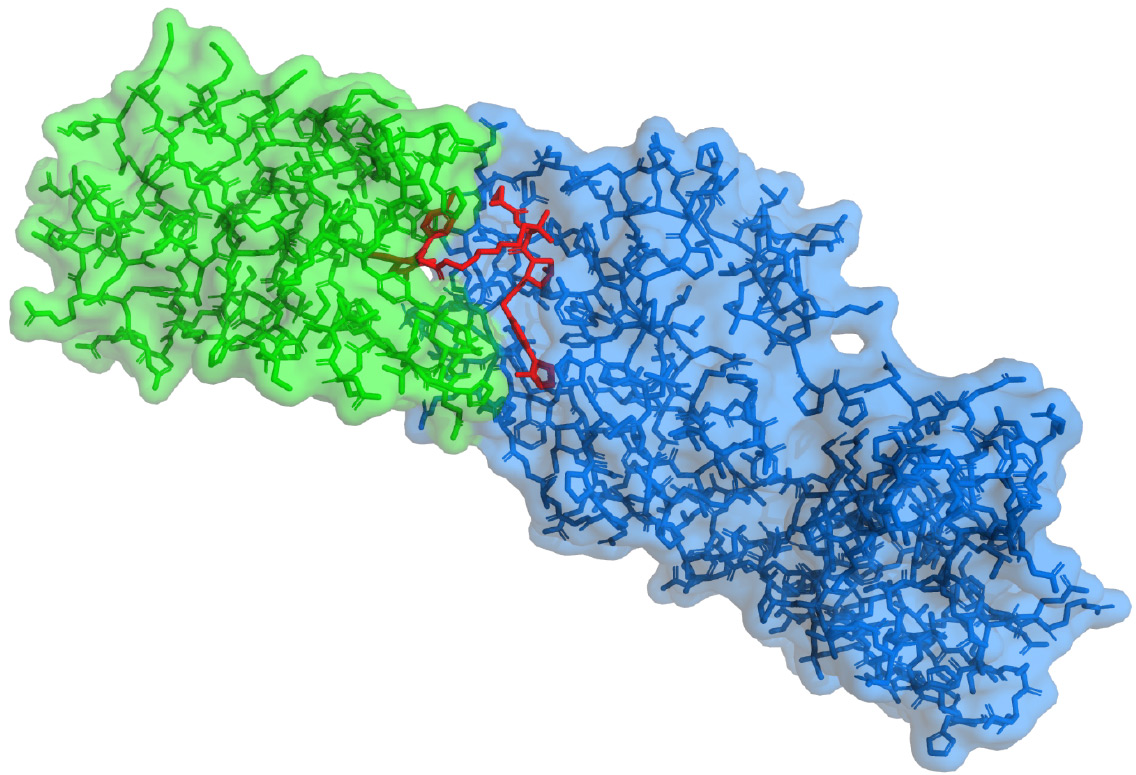
Lastly: ternary complexes in targeted protein degradation (TPD). TPD is a rapidly developing sub-field of drug discovery that revolves around hijacking the cellular process for breaking down proteins and siccing it on a particular protein of interest. The cellular hijacking is mediated through the formation of a ternary complex – a conglomerate of the protein of interest, an enzyme that triggers the protein degradation, and a small molecule interface (the drug). It is therefore of significant interest to develop methods to determine if a ternary complex is formed by the drug, and subsequently to determine the structure of that complex. The first aim is relatively self-explanatory – if no complex is being formed, no degradation occurs, and that particular interface molecule is deemed ineffective. As EPR distance measurements rely on the proximity – the dipolar coupling – of two electron spins, it should be possible to assay complex formation with relative simplicity. Going further, once complexation is confirmed, EPR distance methods are similarly well-suited for determining protein-protein supramolecular arrangements, especially when combined with existing structural information gathered from NMR, X-ray, or CryoEM.

Austin Gamble Jarvi
Applications Scientist
As these conferences continue to build up the context for EPR in various spheres, I continue to be impressed with the work that the scientific community is capable of. Whether it’s biophysics, EPR, drug discovery, or beyond, there is no shortage of exceptional individuals advancing their respective fields, and scientific progress on the whole. I’m excited about the opportunity to engage in the process and help push their work across newfound boundaries, and I’m curious to see how the context shifts as EPR develops further.
With DDC in the bag, the High Q Crew is looking forward to more conferences, more conversations, and more insights in the near future. See you soon!
