Drug Discovery Chemistry 2023 – Context is King
April 20, 2023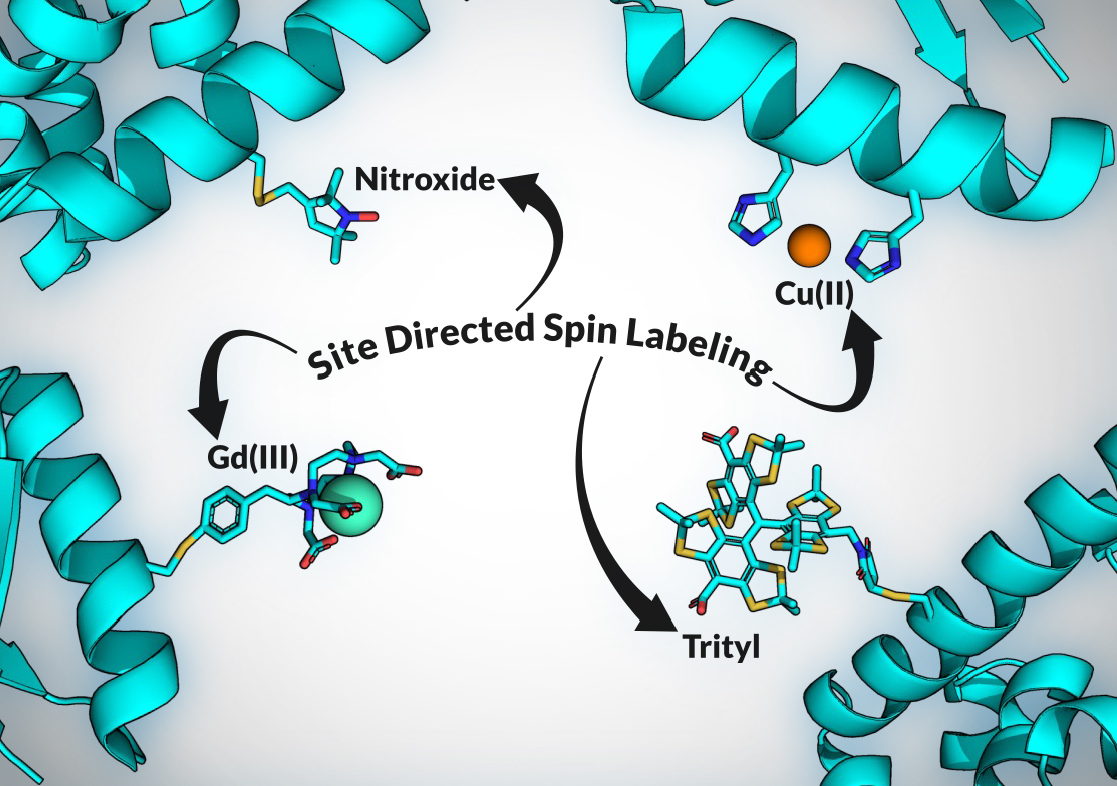
Spin Class: Putting a Label on It
June 22, 2023
Drug Discovery Chemistry 2023 – Context is King
April 20, 2023
Spin Class: Putting a Label on It
June 22, 2023Insights from Austin
The latest insights on EPR technology trends, customer successes and industry best practices
Spin Class: EPR’s Long Distance Relationships
In my first Insights post, wherein I reflected on the Biophysical Society Meeting, I aimed to raise EPR into the common scientific vernacular akin to techniques like CryoEM, which have seen monumental growth and adoption in recent years. I look forward to a time when EPR discussions needn’t start with, ‘Do you know what EPR is?’ However, until that day comes, it may behoove me to introduce you to the technique, providing valuable context and frame of reference for any past and future entries in this series. And so, in this post I will provide a brief overview of EPR, and more specifically, EPR-based distance measurements.
EPR – electron paramagnetic resonance, and sometimes referred to as ESR, electron spin resonance – is a magnetic resonance technique that shares much of the same fundamental underpinnings as the more familiar NMR. Both techniques apply an external magnetic field to polarize a spin system, and both drive spin transitions by applying electromagnetic radiation. The key difference is that EPR, as the name suggests, detects and monitors electron spins rather than nuclear ones. Specifically, EPR detects unpaired electron spins, commonly organic radicals or paramagnetic metal ions. Through a process called site-directed spin labeling (or SDSL, the details and varieties of which are a topic for another time), such unpaired electron species can be site-specifically incorporated into proteins and nucleic acids that would otherwise lack EPR-detectable moieties.
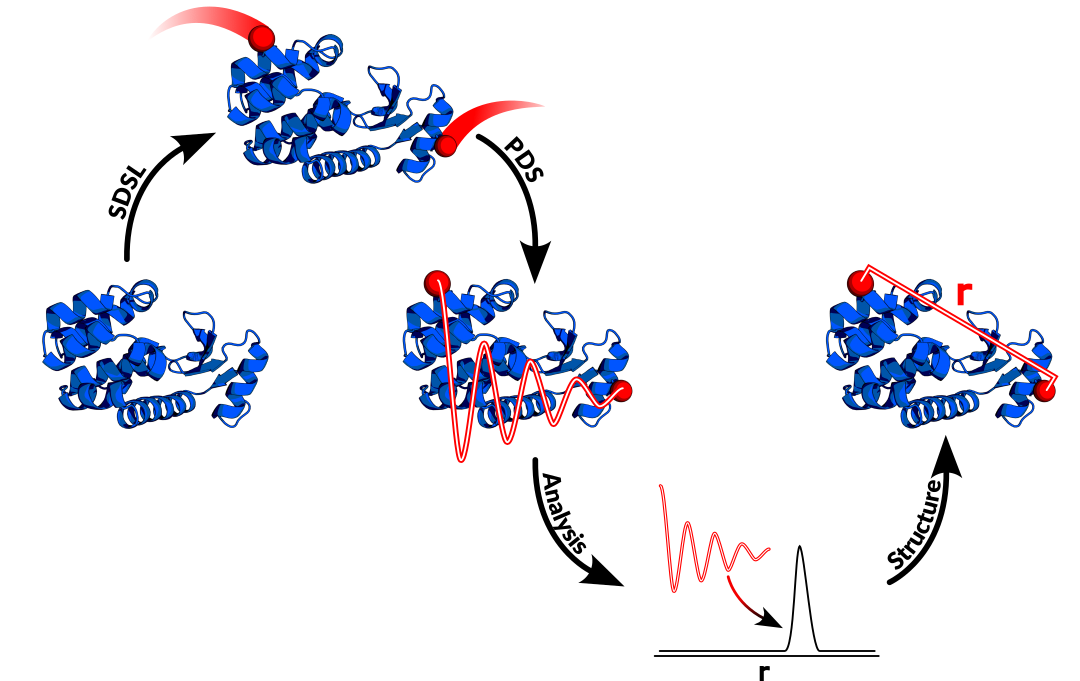 For the purpose of this discussion, EPR experiments can be broadly separated into two categories based on the samples being examined: those containing a single spin label per molecule, and those with multiple labels. Singly spin-labeled species can provide a unique set of information relating to system dynamics and protein topology and structure, but I’d like to focus on the multi-labeled (usually doubly) systems. Electron spins possess an intrinsic magnetic moment, and therefore two unpaired electrons will experience a dipolar interaction. Within the EPR community, a significant amount of effort has gone into developing more efficient ways to extract distance information by isolating this dipolar interaction. Most popularly, pulse EPR, which utilizes nanosecond-scale microwave pulses to manipulate electron spin magnetizations, is employed for this purpose. A number of pulse sequences can be used, each with their own pros and cons (again, a vast topic for another post), but in the end they produce a similar resultant signal – a signal containing a decaying oscillation, the frequency of which is inversely proportional to the distance between the two spins, cubed (r3). In general, this class of experiments is referred to as pulse dipolar spectroscopy (PDS).
For the purpose of this discussion, EPR experiments can be broadly separated into two categories based on the samples being examined: those containing a single spin label per molecule, and those with multiple labels. Singly spin-labeled species can provide a unique set of information relating to system dynamics and protein topology and structure, but I’d like to focus on the multi-labeled (usually doubly) systems. Electron spins possess an intrinsic magnetic moment, and therefore two unpaired electrons will experience a dipolar interaction. Within the EPR community, a significant amount of effort has gone into developing more efficient ways to extract distance information by isolating this dipolar interaction. Most popularly, pulse EPR, which utilizes nanosecond-scale microwave pulses to manipulate electron spin magnetizations, is employed for this purpose. A number of pulse sequences can be used, each with their own pros and cons (again, a vast topic for another post), but in the end they produce a similar resultant signal – a signal containing a decaying oscillation, the frequency of which is inversely proportional to the distance between the two spins, cubed (r3). In general, this class of experiments is referred to as pulse dipolar spectroscopy (PDS).
To extract distance information, the oscillating signal derived from these PDS experiments undergoes analysis. Notably, EPR is an ensemble technique – samples for PDS are flash frozen, capturing the entire retinue of molecular orientations and conformations, all of which contribute to the signal. As such, the distance information derived from PDS is a probability distribution, providing analysts with both a mean, or most-probable distance, and a distribution width which can be used to infer on the molecule’s structure and flexibility. Multiple distinct conformations in the same ensemble may even produce individually resolved distribution peaks. In a perfect world free of experimental noise, extraction of this information would be trivial, but the existence of PDS signal noise necessitates some additional processing to produce reliable data. This namely occurs in a process called Tikhonov regularization, which effectively balances how well the extracted distance data agrees with the raw, noisy signal (the accuracy of the distance data) against the smoothness of the result (such that the noise is not being considered as distance data).
Completion of the above steps leads us to a distance distribution between the spin labels on our selected sites within a protein or nucleic acid of interest. But what can we do with this data? The answer to this question is constantly expanding thanks to the ingenuity and efforts of EPR spectroscopists across the globe. I will briefly touch on some primary use cases for PDS distance information. Often EPR derived distances are used in conjunction with additional structural techniques such as CryoEM, X-ray crystallography, and NMR. In a simple case, EPR is an excellent validation tool for such methods, corroborating structures and determining if sample matrices, preparation methods, or crystallization appreciably impacts molecular conformations. The EPR distribution widths provide insight into molecular flexibility, which is often difficult to attain using other techniques, as they typically report averaged structures. This is all valuable information, but EPR’s true strength lies in its ability to bridge the gaps these other techniques cannot. Its ensemble-capturing nature may detect intermediate conformations that are difficult to isolate alone. Spin labels may be positioned in inherently flexible biomolecular regions that aren’t resolved by other means. As well, EPR is not limited in protein size, and can easily be applied to proteins too large for NMR, or too small for CryoEM. EPR distance constraints may also be coupled with in silico methodologies, like molecular dynamics simulations, to investigate protein dynamics, motions, and the mechanisms of conformational change. This is not even to mention using EPR constraints in conjunction with the burgeoning field of AI predictions like AlphaFold as a means of validation and beyond.

Austin Gamble Jarvi
Applications Scientist
At this point I must stop myself, before the blog is categorized as an essay. In truth, multiple theses could be written (and have been) about each of the above paragraphs. EPR distance techniques are a deep and varied topic, only the surface of which I have begun to scratch. But the basics I’ve discussed thus far should provide you with a solid, qualitative foundation on which to put the rest of my content into context, or serve as a jumping off point to a deeper discussion. As this series progresses I look forward to exploring and sharing the many facets of EPR with you. Until then!
